![PDF] Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin's lymphoma: correlation of complete response, time-to-event and overall survival end points | Semantic Scholar PDF] Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin's lymphoma: correlation of complete response, time-to-event and overall survival end points | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/1b7c7d2ac6a943299f03859ee0550a5a659900c1/4-Table2-1.png)
PDF] Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin's lymphoma: correlation of complete response, time-to-event and overall survival end points | Semantic Scholar

Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study - ScienceDirect

Supplemental Materials for iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics - The Lancet Oncology
KAZIA PRESENTS FURTHER PAXALISIB AND CANTRIXIL DATA AT AACR, REINFORCING POSITIVE EFFICACY SIGNALS FOR BOTH DRUGS

Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria | Clinical Cancer Research

Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors | Nature Communications
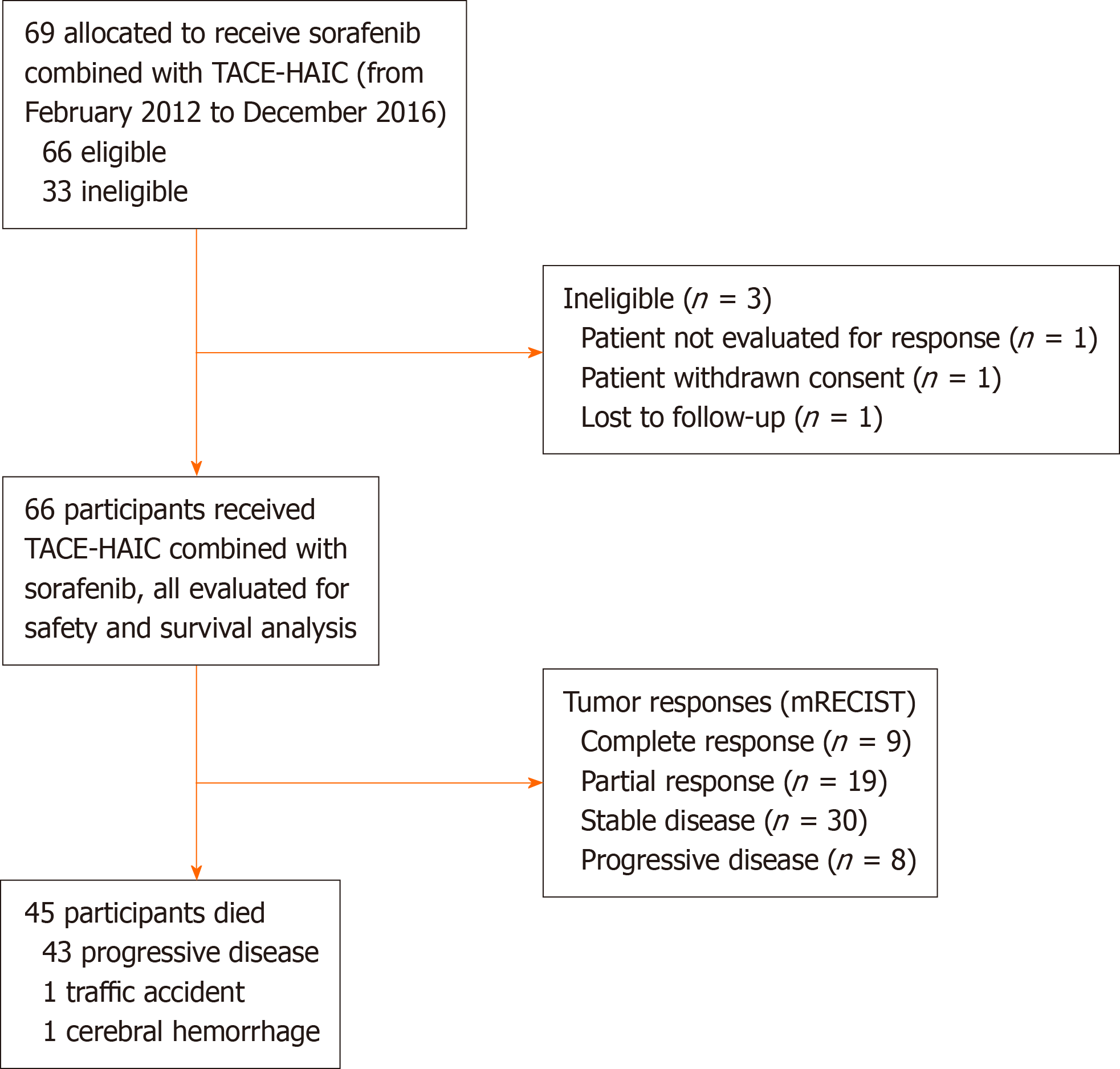
Sorafenib combined with embolization plus hepatic arterial infusion chemotherapy for inoperable hepatocellular carcinoma

Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry - Annals of Oncology
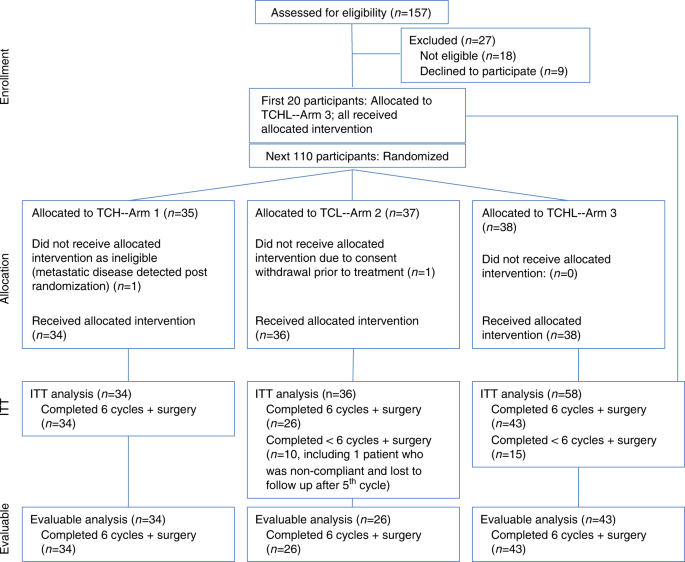
Pathologic and molecular responses to neoadjuvant trastuzumab and/or lapatinib from a phase II randomized trial in HER2-positive breast cancer (TRIO-US B07) | Nature Communications





![PDF] Evaluation and monitoring of response to therapy in multiple myeloma. | Semantic Scholar PDF] Evaluation and monitoring of response to therapy in multiple myeloma. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c7433976b18e415f228f8d8e6521bba0c26bbc89/2-Table1-1.png)